Gene Therapy Drug Interaction Checker
How This Tool Works
This tool helps you understand potential interactions between your current medications and gene therapy. Based on the article, viral vectors can alter liver enzymes that process many drugs. Select your medications below to see potential risks.
Gene therapy isn’t just another treatment option-it’s a fundamental rewrite of how medicine works. Instead of managing symptoms with pills or injections, it tries to fix the root cause: faulty genes. But this power comes with hidden risks, especially when patients are also taking other medications. The interactions between gene therapy and drugs aren’t like typical drug-drug clashes. They’re unpredictable, delayed, and sometimes life-threatening. And we’re only beginning to understand them.
Why Gene Therapy Is Different From Every Other Drug
Most drugs work temporarily. You take a pill, it circulates, does its job, and leaves your body in hours or days. Gene therapy is permanent. Once the therapeutic gene is delivered into your cells, it can keep working for years-or your entire life. That’s why safety rules don’t apply the same way. A side effect that shows up a week after a regular drug might not appear until five years after gene therapy. And by then, it’s too late to undo the damage. The biggest danger comes from the delivery system: viral vectors. These are modified viruses-like cold viruses or harmless adenoviruses-that carry the new gene into your cells. They’re engineered to be safe, but they still look like invaders to your immune system. In 1999, an 18-year-old named Jesse Gelsinger died after receiving gene therapy for a rare liver disorder. His body mounted a massive immune reaction to the adenovirus vector. His organs shut down. It wasn’t a mistake in the gene itself-it was the delivery system. And that’s just the beginning.When the Vector Turns Against You
Viral vectors don’t just trigger inflammation. They can mess with your body’s drug-processing machinery. Your liver uses enzymes called cytochrome P450 to break down most medications-antibiotics, painkillers, blood thinners, antidepressants. When a viral vector enters your bloodstream, your immune system releases cytokines. These inflammatory signals can turn those liver enzymes on or off. Suddenly, a drug you’ve taken safely for years becomes too strong-or doesn’t work at all. Imagine someone on warfarin, a blood thinner, gets gene therapy for a blood disorder. A week later, their INR (a measure of blood clotting) spikes. Doctors assume they missed a dose. But it’s not that. The gene therapy vector triggered an immune response that suppressed CYP2C9, the enzyme that breaks down warfarin. The drug built up to toxic levels. No one knew to check for this. It wasn’t in the drug label. It wasn’t in any textbook. It was invisible until it was too late.Off-Target Effects and Hidden Risks
Gene therapy isn’t always precise. The vector might deliver the gene to the wrong cells. Liver cells? Maybe. But what about heart cells? Brain cells? Or stem cells that multiply for life? In early trials for severe immune deficiencies, five children developed leukemia because the therapeutic gene landed right next to a cancer-causing gene called LMO2. The vector didn’t cause cancer directly-it activated it. That’s called insertional mutagenesis. And it can take years to show up. Now imagine a patient gets gene therapy for a muscle disorder. The vector accidentally modifies liver cells. Those cells start producing a protein they never should. That protein changes how the liver metabolizes statins, the cholesterol drugs. The patient ends up with muscle damage from statin toxicity. No one connects the dots. The gene therapy was given three years ago. The doctor doesn’t even remember it.Long-Term Monitoring Isn’t Optional-It’s Essential
The FDA now requires 15 years of follow-up for gene therapies that integrate into your DNA. That’s not a suggestion. It’s a rule. Why? Because some risks don’t show up until adolescence, middle age, or even later. A child treated for a rare disease at age 5 might develop a tumor at 22. Or their immune system might suddenly start rejecting the therapeutic gene at 40. Or their liver enzymes might shift after a new medication is added. This isn’t just about tracking side effects. It’s about understanding how drugs behave differently over time in gene therapy patients. We don’t have databases of thousands of people on gene therapy and their full medication histories. We’re building that database one patient at a time-and many are already past the five-year mark without proper monitoring.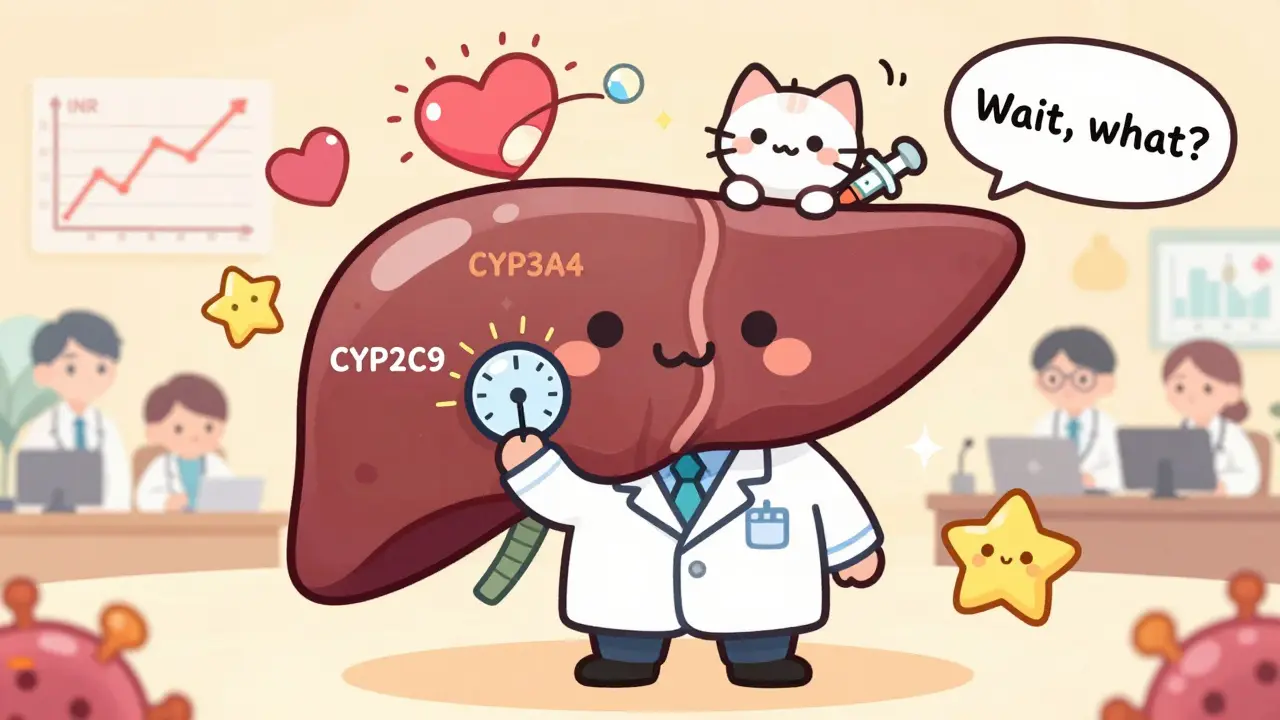
AAV Therapy: The New Normal With Old Problems
Today, most gene therapies use adeno-associated viruses (AAVs). They’re less inflammatory than adenoviruses. But they’re not harmless. Up to 70% of adults have pre-existing antibodies to common AAV serotypes. That means their immune system recognizes the vector before it even delivers the gene. The therapy fails-or triggers a dangerous immune reaction. And if the patient is on immunosuppressants for another condition? That changes everything. The drug might block the immune response enough to let the therapy work… but also let a hidden virus reactivate. Different AAV types target different tissues. AAV9 goes to the brain. AAV8 to the liver. AAV6 to the lungs. If you’re getting AAV9 for spinal muscular atrophy and you’re also on a drug that crosses the blood-brain barrier, you might get unexpected neurological side effects. No one tested that combo. No one will until someone gets hurt.Who Gets Left Out?
Most gene therapy trials involve small, homogenous groups-mostly white, middle-class children with rare diseases. But real patients are diverse. They’re older. They have diabetes. They take blood pressure meds. They’re on antidepressants. Their genes vary. Their livers work differently. Their immune systems are worn down. We don’t know how gene therapy interacts with common drugs like metformin, lisinopril, or sertraline in these populations. We assume the data from young, healthy trial participants applies to everyone. It doesn’t. And when a 65-year-old with kidney disease gets gene therapy and then takes a common antibiotic, the result could be catastrophic. But we won’t know until it happens.The Unspoken Risk: Transmission
Here’s something most people don’t talk about: gene therapy can spread. Not like a cold. But through bodily fluids. If a patient sheds the viral vector in saliva, blood, or semen, a partner, child, or caregiver could be exposed. They didn’t consent. They didn’t get screened. They’re not under medical supervision. And if they’re on medications? They could get a silent, unmonitored gene therapy dose. The FDA requires companies to prove this won’t happen. But proving a negative is hard. And some vectors are designed to be stable in the environment. We’re playing with fire and pretending it won’t spread.What Needs to Change
We need mandatory drug interaction logs for every gene therapy patient. Not just a checkbox on a form. A living record: what drugs they take, when they start, when they stop, what labs change. We need pharmacists involved-not just doctors. We need registries that track patients for 20 years. We need research into how AAV serotypes interact with specific drugs. We need to stop assuming gene therapy is just another pill. Right now, the system is built for short-term fixes. Gene therapy demands long-term thinking. It’s not just about curing a disease. It’s about managing a lifetime of biological change-and the drugs that come with it.What Patients Should Ask
If you’re considering gene therapy, ask these questions:- What viral vector is being used, and what tissues does it target?
- What drugs am I currently taking that could interact with this therapy?
- Will my liver enzymes be monitored before, during, and after treatment?
- How long will I be followed, and who will track my medications?
- What happens if I need surgery or start a new medication five years from now?
- Is there a registry I can join to help track long-term effects?
These aren’t just good questions. They’re survival questions.
It’s Not Science Fiction-It’s Today
Gene therapy isn’t coming. It’s here. Over 30 therapies are approved worldwide. More are in late-stage trials. But our drug safety systems haven’t caught up. We treat gene therapy like a new drug. It’s not. It’s a new biology. And until we treat it that way, patients will keep getting hurt-not because the science failed, but because we didn’t listen to what the science warned us.Can gene therapy cause cancer years after treatment?
Yes. In early trials for immune disorders, gamma-retroviral vectors inserted the therapeutic gene next to the LMO2 proto-oncogene, triggering leukemia in five children. This was not a flaw in the gene itself, but in how the vector integrated into the genome. Modern vectors like AAVs are less likely to cause this, but the risk isn’t zero. Long-term monitoring for 15 years or more is now required for integrating therapies.
Do I need to stop my medications before gene therapy?
Sometimes-but not always. The decision depends on the vector, your current drugs, and your immune status. Some medications, like corticosteroids, may be given to suppress immune reactions to the viral vector. Others, like blood thinners or statins, may need temporary pauses if the therapy affects liver enzymes. Never stop a medication without consulting your care team. There’s no universal rule-it’s personalized based on your therapy and health history.
Can gene therapy affect how my body processes common drugs like ibuprofen or antibiotics?
Absolutely. Viral vectors trigger immune responses that can turn liver enzymes on or off. These enzymes (like CYP3A4 and CYP2C9) break down most drugs. If they’re suppressed, drugs like ibuprofen, statins, or antibiotics can build up to toxic levels. If they’re overactive, the drugs may not work. This interaction can happen weeks or months after gene therapy and is often missed because it’s not listed on drug labels.
How long should I be monitored after gene therapy?
For gene therapies that integrate into your DNA or can remain dormant (like HSV-based vectors), the FDA requires 15 years of follow-up. This includes regular blood tests, imaging, and medication reviews. Even for non-integrating therapies like AAV, experts recommend at least 10 years of monitoring because delayed immune reactions or off-target effects can appear years later.
Is gene therapy safe if I’m on multiple medications for chronic conditions?
We don’t have enough data to say for sure. Most trials exclude patients on multiple medications to reduce variables. But real patients often have diabetes, heart disease, or depression. The interactions between gene therapy and these drugs are largely unknown. If you’re on chronic meds, ask your team if your specific combination has been studied. If not, proceed with extreme caution and insist on long-term monitoring.
Can my family members get gene therapy from me after treatment?
It’s rare, but possible. Some viral vectors can be shed in bodily fluids like saliva, blood, or semen. While most therapies are designed to prevent this, the risk isn’t zero. If transmission occurs, a family member could receive an unmonitored, unconsented gene therapy dose-especially dangerous if they’re on medications. Always ask your care team about shedding risks and what precautions to take with close contacts.



Comments
This is terrifying. I had no idea gene therapy could mess with my blood thinners like that. My aunt just got treated for a blood disorder, and she’s still on warfarin. I’m gonna call her doctor right now. 🤯
We treat gene therapy like a magic bullet, but it’s more like installing a new operating system on a 20-year-old laptop and expecting no crashes. The body isn’t a lab. It’s a messy, evolving ecosystem. We’re playing god with patch notes we haven’t even finished writing.
Thank you for writing this. I’m a nurse in a pediatric oncology unit, and we’ve got two kids on AAV therapies right now. Their parents are so hopeful, but no one talks about the long-term med risks. I’ve started keeping a shared med log with their families-simple Google Sheet. If this helps even one person avoid a toxic reaction, it’s worth it. 💪
It is imperative to recognize that the cytochrome P450 system is not merely a metabolic pathway-it is a dynamic, immune-modulated regulatory network. The suppression or induction of CYP2C9 and CYP3A4 following viral vector administration constitutes a pharmacokinetic paradigm shift. Clinical protocols must be revised accordingly, with mandatory pre- and post-treatment enzyme profiling.
Wow. Just... wow. I read this and I'm like-why are we even doing this? It's like giving someone a nuclear reactor and saying 'don't worry, we tested it for a week.' This isn't medicine. It's a gamble with human lives. And the FDA? They're just rubber-stamping it. I'm not even sure I'd trust my own doctor with this.
AMERICA NEEDS TO STOP BEING SO CAUTIOUS!! 🇺🇸🔥 This is the FUTURE. We’re talking about CURING DISEASES-PERMANENTLY. If some guy gets a bad reaction because he was on ibuprofen? TOO BAD. We can’t let fear stop progress. These people are LUCKY to even get this chance. Stop whining and start funding more trials!! 🚀💊 #GeneTherapyWins
Okay, imagine your liver is a nightclub bouncer. For years, it’s been letting in ibuprofen, statins, and antibiotics with a nod. Then-BAM-gene therapy crashes the party like a rave DJ with a Tesla coil. Suddenly, the bouncer’s got a panic attack, slams the door on half the meds, and lets in a whole new crowd of chaos. And no one told the guests. That’s not science. That’s a horror movie written by a tired pharmacist at 3 a.m.
AAV serotype-specific immune modulation → altered hepatic CYP expression → pharmacokinetic nonlinearity. Standard monitoring protocols are obsolete. Requires pharmacogenomic integration. No exceptions.
My mate’s daughter had SMA gene therapy last year. She’s doing brilliant. But the hospital? Zero follow-up on meds. She’s on a new antibiotic for a chest infection last month, and no one asked if she’d had gene therapy. I nearly had a heart attack. This isn’t just oversight-it’s negligence.
How quaint. You think this is new? The pharmaceutical-industrial complex has been pushing ‘miracle cures’ since the 1950s. Thalidomide. Vioxx. Now gene therapy. The only difference? Now they’re doing it to children. And we’re supposed to be grateful? Please. The real crime isn’t the science-it’s the fact that we’ve stopped asking who profits.